Your new ally for fighting depression
SAFE. PROVEN. EFFECTIVE
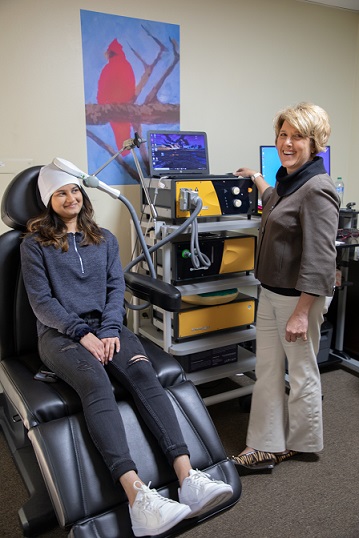
Transcranial magnetic stimulation (TMS) is a safe, non-invasive, and non-drug procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS has been FDA-approved for the treatment of depression since 2008, and is typically used when other depression treatments haven't been effective.
Transcranial Magnetic Therapy (TMS) is a non-drug, non-invasive form of brain stimulation therapy that treats depression at the source.
TMS is the most advanced form of FDA-approved treatment for major depressive disorder and obsessive compulsive disorder (OCD).
Generally, TMS is considered safe and well-tolerated. The majority of patients improve during treatment and continue to feel better after treatment is complete.
TMS is an outpatient treatment, administered without the need for anesthesia, that allows patients to return to normal activity directly following treatment.
Many factors figure into your TMS treatment plan for depression. If you want to know if TMS is a good option for you
You can resume your normal daily activities immediately after each TMS session.
TMS is non-invasive, which means the patient is fully conscious during the treatment. Some patients report feeling a tingling sensation in their scalp during treatment as the stimulation occurs or a slight headache after the treatment is over. Other sensations include a mild twitching around the facial or eye area during treatment, though this subsides immediately after the session has ended. Overall, TMS is relatively painless, and any physical sensation felt during or following a session is mild and brief.
TMS can be combined with most antidepressant medications, but it's important to discuss this matter with a qualified and experienced mental health practitioner before making any decisions regarding antidepressants and other medications. So, your current medications and their compatibility will be evaluated during your pre-treatment evaluation appointment. You're welcome to continue any psychotherapy you're currently undergoing, or if you're interested, we can offer you psychotherapy and medication management.
Every patient is different, and the time it takes to notice results from TMS therapy is different for each individual as well. Some patients report feeling a slight improvement in their symptoms after two weeks of treatment, while others do not report noticeable changes until weeks four or five. If it takes longer to notice symptom relief using TMS compared to someone else, this does not mean that the treatment is ineffective. Factors that may affect your time to results with TMS include age, brain activity prior to treatment, the level of treatment resistance, and personality characteristics.
TMS has been proven to provide long-lasting results, but vary from patient to patient. TMS is a durable treatment for depression with sustained responder rates of 50% up to 1 year after a successful induction course of treatment. In some cases, patients may need a maintenance session of TMS.
TMS is a non-invasive form of brain stimulation and does not require surgery or sedation with anesthesia. TMS is considered safe and is generally well-tolerated. However, it can cause some side effects. Common side effects include headache, scalp discomfort at the site of stimulation and/or tingling or twitching of facial muscles. These side effects are mild to moderate, improve after the treatment session and diminish over the course of TMS therapy.
Some patients may experience transient hearing problems immediately following treatment. Patients are given ear plugs during the treatment session to prevent any adverse effect on hearing from the noise produced by the TMS machine.
The most serious risk of TMS is seizures. However, the seizure risk is extremely low (0.1% to 0.5%) and comparable to that for anti-depressant medications. The seizure described were self-limited, required no medications and did not recur. Our treatment guidelines have incorporated safety precautions to minimize the seizure risk. Patients with underlying bipolar disorder may develop mania.
Transcranial magnetic stimulation (TMS)treatment for depression involves delivering repetitive electromagnetic pulses, so it's called 'repetitive TMS' or 'rTMS'. In a clinical setting, there is no difference. All TMS therapy programs that are available to patients for the treatment of depression are technically rTMS, even though physicians typically drop the 'r' and refer to them as 'TMS.'
TMS and ECT are both non-invasive neuromodulation procedures used to treat individuals with depression who do not respond to standard medication(s). However, ECT is associated with a number of safety risks, adverse effects and logistical constraints.
ECT (also referred to as "shock therapy") uses an electric current that passes between two electrodes placed on the scalp to induce a "generalized seizure" while the patient is under general anesthesia. A course of ECT involves a series of treatments delivered over several days to weeks. Unlike ECT, TMS induces electrical activity in specific regions of the brain using pulsed magnetic fields and does not require anesthesia. Patients are fully awake and alert during the TMS procedure, and able to converse.
ECT is generally administered in a hospital setting while TMS is an outpatient procedure done in an office setting. Patients do not need sedating or muscle relaxant medications for TMS procedure and are able to drive themselves home after the treatment session.
The immediate side effects of ECT can be headaches, muscle pain, nausea, and confusion requiring observation in the hospital. Patients may experience mild to moderate scalp discomfort after TMS procedure but this generally lasts for a short duration. Some patients receiving ECT may develop "short term" memory loss (inability to remember the new information) and/or "long term" memory loss(inability to recall events from the distant past). Rarely, impairment of long term memory can be permanent. Memory loss has never been reported in patients receiving TMS treatment.
Unfortunately, yes. TMS is not a guaranteed cure, and it does not get rid of symptoms in everyone. All available treatments in psychiatry work for some, but not all, patients. TMS is not an exception. Studies have shown that psychotic depressed patients, patients who have failed a course of ECT, and patients with severe comorbid mental disorders like schizophrenia, post-traumatic stress disorder or panic disorder generally do not benefit from the traditional depression TMS protocol. Each case needs to be assessed individually.
Patients can schedule an appointment with the psychiatrist for TMS therapy evaluation by CALLING our office during normal business hours (8AM to 5PM, Mon-Fri) or by filling out FREE TMS screening form